The periodic table of the elements is almost as old as The College of Wooster, and I am a big fan. As we approach next year’s sesquicentennial of Dmitri Mendeleev‘s 1869 periodic table, I present a modest addition to the over 600 known periodic tables, which include 2D, 3D, and even 4D designs!
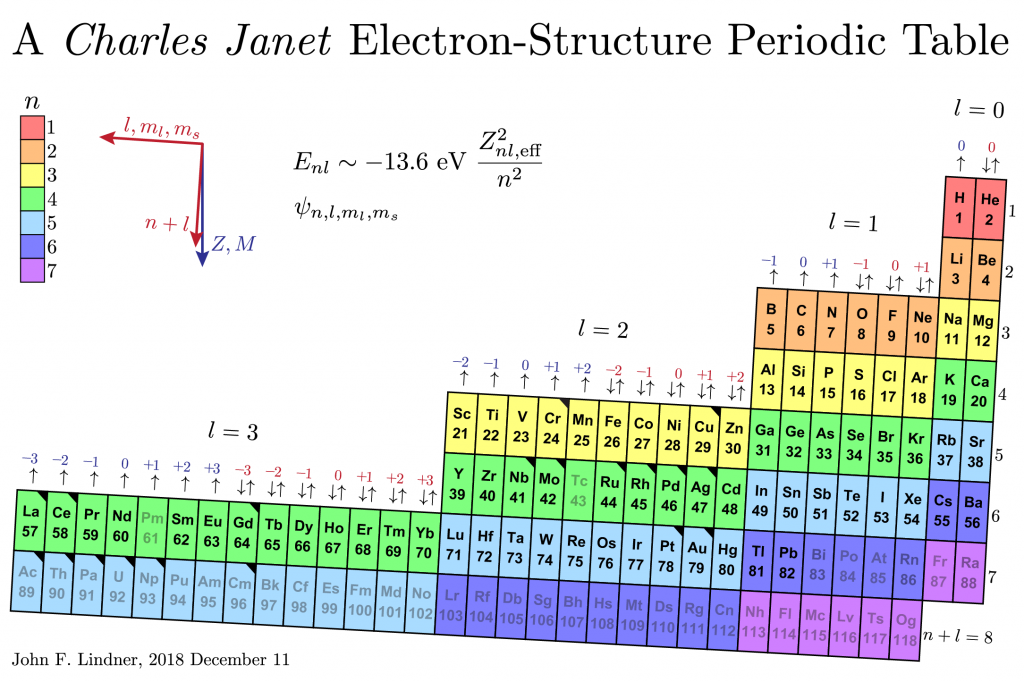
I wanted the table to reflect an element’s electron configuration, its energy and angular momentum quantum numbers n, l, m_l , m_s, and its mass M and nuclear charge Z. I also did not want to alienate viewers familiar with the classic twentieth-century short-form periodic table, but problems with the short form include gaps and jumps in the presentation and the way the lanthanide and actinide elements appear as footnotes to the main table.
I first created a large electron-configuration periodic table for the Taylor 111 physics lecture hall about 15 years ago. Recently, I learned that French amateur scientist Charles Janet (pronounced “sharl shuh nay”?) first created such designs circa 1930. I have a paper Janet table rolled into a spiral and a wooden one arranged like a stepped layer cake.
In my 2D version, rainbow colors code the principle quantum number n. The levels and blocks tilt slightly to emphasize that lower means larger mass M and charge Z. Lutetium and lawrencium are directly below scandium and yttrium, as they should be, not appended to the end of the lanthanide-actinide footnote. The placement of helium is nontraditional, and if it makes you uncomfortable, I feel your pain, but chemistry literature exists to support it.
Black corner triangles tag elements with exceptional electron configurations, the lightest being chromium and copper. Grayed symbols indicate elements without stable isotopes: technetium, promethium, and all elements heavier than lead. (Bismuth and uranium are nearly stable having isotopes with half-lives much longer than the age of the universe and about the age of Earth.)



I love the nerve that brought you to tilt the PT! The first I’ve seen.
I’d appreciate it if you would accept a kit for assembly of my periodic ‘table’ for your appraisal and/or amusement.
Would I best send it to Wooster College under your name?
Sincerely,
Roy Alexander 773.271.0318 roy@AAEinfo.usa
P.S. Details may be found at 3dperiodictables.com, chemicalelementsystem.com, and allperiodictables.com.
Hi Roy,
Thanks for your feedback!
Yes, please send me one of your periodic table kits to the address below, which I will assemble and display in my office.
Best regards,
John
@@@@@@@@@@@@@@@@@@@
John Lindner
Physics Department
The College of Wooster
Wooster OH 44691
@@@@@@@@@@@@@@@@@@@